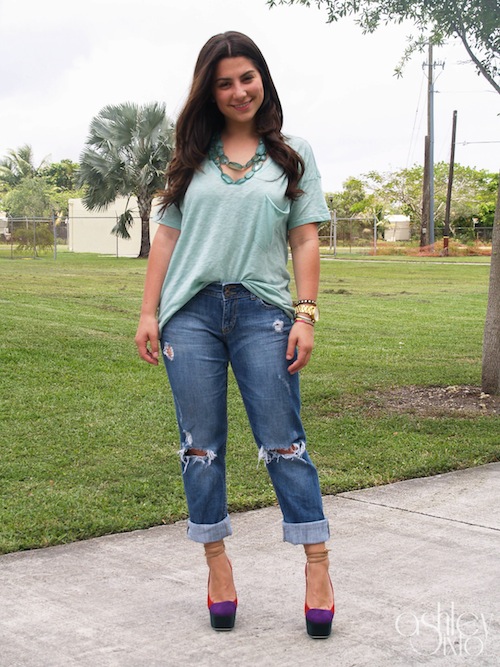





top: Forever 21. necklace: Forever 21 (similar). jeans: Express. heels: YSL c/o Saks. watch: Nixon. Bracelets: (left to right) Forever 21, David Yurman, c/o N&K Designs.
Christmas came real early for me this year. I entered an Instagram competition with Saks Fifth Avenue a few weeks ago and WON $1000 Gift Card! It was one of the most surprising and jaw dropping experiences ever to find out I had won. But once it got down to making my purchase or purchases, I freaked out. As many of you know, I’m a woman who maximizes her purchases and never splurges. I had no idea what to do, but at the same time I did. I wanted a new pair of shoes! After all those steal finds, it was my turn to finally purchase the splurge! Walking into the Saks shoe department was like walking into shoe heaven, no joke. All the brands I had dreamed of owning, were there at my fingertips ready to be told “I’m taking you home!” After trying on pair after pair, nothing made my heart go bonkers like these heels. Immediately when I put them on, I knew these were the ones. So I got them and I don’t want to take them off. Us women and our shoes….
Now in the spirit of giving I’ve teamed up with Nani and Kaki Lopez from N&K Designs to give away one of their neon rhinestone bracelets (like the one I’m wearing above)! They’re so fun and just the perfect thing to make your arm candy sparkle. Want to win? Here’s the catch:
Like AshleySixto.com and N&K Designs on Facebook & leave a comment here with what your favorite AshleySixto.com post is!
Giveaway will close on 5/22 and the winner will be announced on Wednesday, 5/23… GOOD LUCK !!!
Contest is now closed. Congrats to comment # 17 – Alejandra! You are now a new proud owner of an N&K Designs Bracelet! I’ll be sending over an email to you now 🙂
You have an awesome style and those shoes are so hot! But I can never wear such high heels!!
Hope I win the giveaway
xox
Shizzy
Oh, my fav product has to be Beatriz Necklace from N&K
x
I have to admit that I follow you on instagram and go on your website before an outing so that I can be up to date with the latest fashion statements. I LOVE your splurge vs. steal posts but I have to say that my favorite post right now has to be “May Cravings: Just add Water,” since it gives ideas for cute and affordable bathing suits perfect for the Spring and upcoming Summer season. Thank you for your fashion tips! Hope I win so that I could add more to the N&K Design arm party I have thus far. 🙂
Favorite post- April 10, 2012.
LOVE the shoes. And that “Rico Suave” dude gave your shoot tons of character. He needs practice behind the camera though. Obviously, doesn’t run in the family….
<3
I love all of the posts on here but my favorite so far has been “Spotty Dotty” on April 23rd. I loved how you dressed up your cutoffs with a fun blouse (:
My favorites are a tie between mint condition and Ashley goes to prep school!!! I love all the posts and I use all your tips and links
super cute look!! love your jeans 🙂
http://www.kellydillon.com
Hi doll!
———————— I’m now a FB fan of you both under: Nicole O.
————————- My fave post of yours is the Ssssssnake-Pants. The chambray looks really awesome with the snake print. Love the whole outfit.
Thank you for the chance!
Hey Ashley !
I love your cite it has been an inspiration ! I really love the outfit that was featured on asos . I also liked your night time attire in the fair pictures (I really do lol ) I don’t know the names of the posts right now and my bluberry sucks
Love you !
Franchenstein
My favorite post is from March 19th…Punky Brewster.
You’re doing great Ash!
My favorite post was “The Canvas Backpack” I am always on the GO so to have a backpack designed for us busy bee’s is awesome! I also enjoyed the green pants you were wearing and the simplicity of a navy blue top! Your sense of style is genius… and YES! I have become a fan of N&K.
I’m obsessed with you so I should win. ❤
oh and my favorite posts are splurge vs. steal. ^_^
P.s. with your guidance im thinking I should make a chunky girl fashion blog. ^.^
“A carrie barhsaw moment ” its the name of the post i like the most about this blog!! Well to be sincere, i’m following you on IG since couple weeks ago!! And i can say i really love ur style!! Im not s very fashion person but i like to see others that are pretty like you!! So as u can see i’m new follower of ur blog!! Hope i can win !!!
A carrie bradshaw moment!! Its the name!!! Sorry !! Lol
My favorite was splurge vs steal. I’m always looking for steal without having to spend the big bucks. While still looking stylish of course! Short and sweet post.
I liked your page and N&K designs on Facebook.
Great blog.
Im loving the shoes. So gorg!
Hi Ashley, I love your blog! My favorite post was funky florals, what can I say, I’m a flower child. I actually went to the New York H&M and bought the same top! (same print but different style), now all i need are some green pants!
Date night came out awesome. Then again, everything looks awesome on you 😉 love you BFFN !!!!!
huh, so cute heels, wish I could wear something like these some time
Pingback: Splurge VS Steal: YSL Colorblock Platforms |